Non graphite carbon tends to graphitize at high temperatures. Based on the difficulty of graphitization, precursors can be divided into two types: easily graphitized carbon (soft carbon) and difficult graphitized carbon (hard carbon).

Carbon obtained from coal tar pitch, petroleum pitch, anthracene, etc. belongs to easily graphitized carbon, while carbon obtained from cellulose, phenolic resin, furan resin, etc. belongs to difficult graphitized carbon. Non graphite carbon materials have large interlayer spacing and disorder, which are conducive to the de intercalation of Na+, making them the most studied type of material. It was found that the mesophase carbon microspheres prepared at 750 ℃ had a certain sodium embedding property. After heat treatment at 950 ℃ under argon protection, Resorcinol formaldehyde resin pyrolysis carbon microspheres were prepared (see figure).
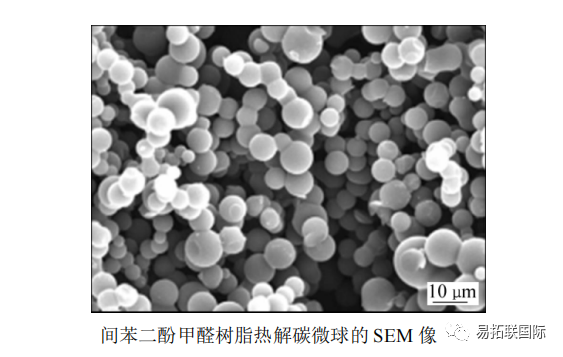
This carbon microsphere has a high degree of disorder, low specific surface area (3m2/g), and a reversible sodium storage capacity of 285mA · h/g. Below 0.2V, a capacity of 247mA · h/g can be obtained.
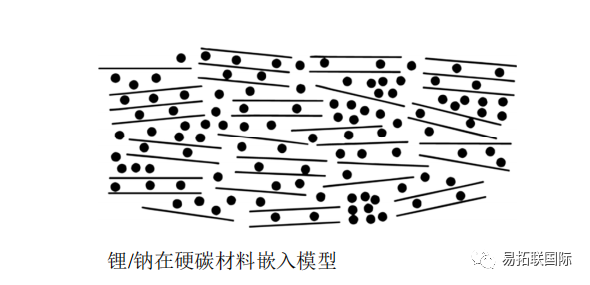
The MCMB negative electrode material was prepared by hydrothermal treatment of glucose at 180 ℃ and then heat treatment at 1000 ℃. The results showed that its sodium intercalation ability was close to that of lithium intercalation. When charged and discharged at a low rate of C/80, the reversible capacity was 300mA · h/g, but the cycling stability was poor and the decay was fast. The lithium and sodium intercalation models are shown in the figure.
The charging and discharging properties of hard carbon in 1mol/L NaClO4 Ethylene carbonate (EC), propylene carbonate (PC) and butyl carbonate (BC) electrolytes were studied (see figure).
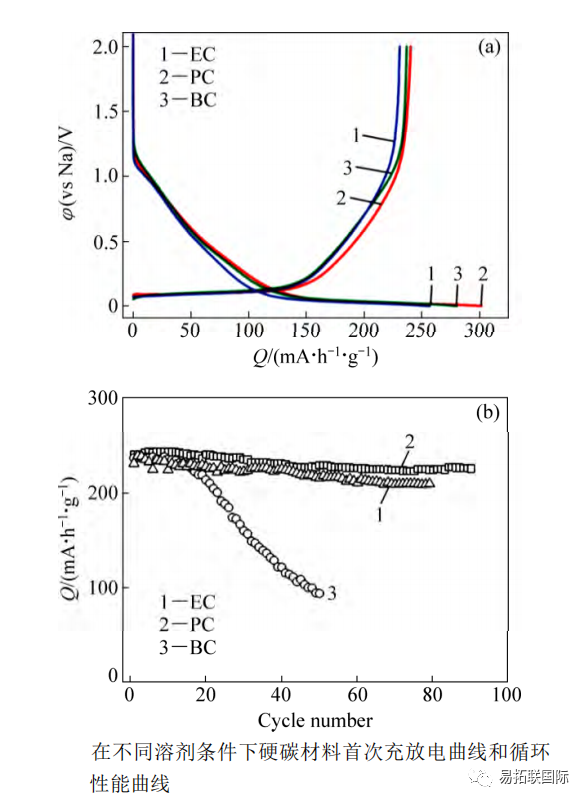
When the current density is 25mA/g, the first reversible capacity can reach over 220mA · h/g. In EC and PC electrolytes, the cycling performance is relatively stable, while in BC electrolytes, the reversible capacity sharply decreases after 20 cycles. And they also prepared full batteries with hard carbon negative electrodes and NaNi0.5Mn0.5O2 positive electrodes. At a current density of 300mA/g, the first reversible capacity reached 250mA · h/g, and after 50 cycles, the reversible capacity could still reach over 150mA · h/g. X-ray diffraction analysis shows that the interlayer spacing of hard carbon increases when Na+is embedded, while it is opposite when it is removed, indicating that Na+can be reversibly intercalated between hard carbon layers. Similar results were also obtained through nuclear magnetic resonance research. Template carbon was prepared using mesophase carbon asphalt using nano porous silicon as a template (see figure).
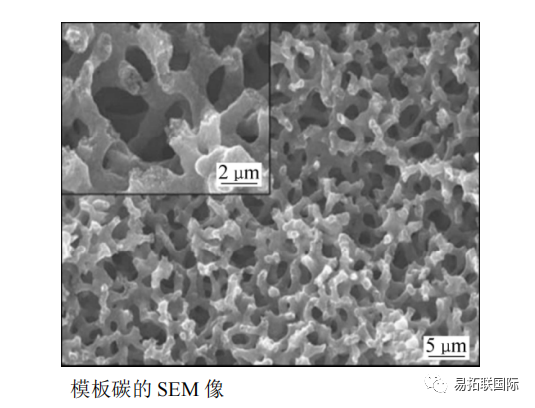
At the C/5 ratio, the reversible capacity exceeds 100mA · h/g, while at the 5C ratio, the capacity is still greater than 100mA · h/g, demonstrating excellent rate discharge performance. Hollow carbon nanowire cathode materials were prepared using hollow Polyaniline nanowires as precursors. After 400 cycles, the reversible capacity was 251mA · h/g, the capacity retention rate was 82.2%, and the reversible capacity was 149mA · h/g when the current density was 500mA/g. It is believed that the high capacity, good cycling performance, and high current performance of hollow carbon nanowires are due to the large interlayer spacing and short diffusion distance. The influence curve of interlayer spacing on the embedded energy consumption of Na+and Li+(see Figure).
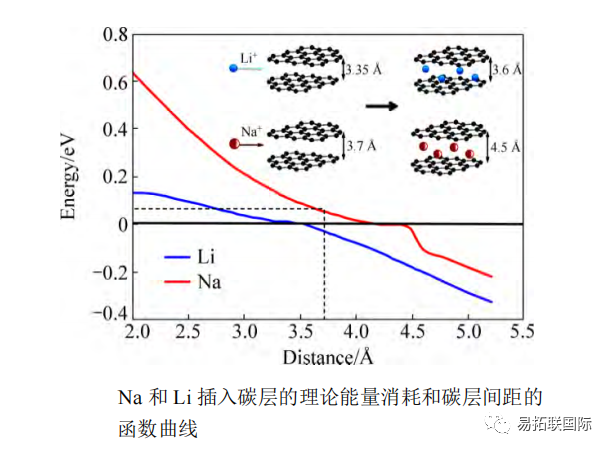
The slope of Na's curve is larger than that of Li's. When the interlayer spacing of carbon is 0.335nm, the energy consumption of Li+is low at 0.03eV, making it easy to embed between carbon layers; The energy consumption of Na+is high at 0.12eV, making it difficult to embed between graphite layers. As the interlayer spacing increases, the embedding energy consumption of Na+decreases, making it easier for Na+to be embedded. They believe that the minimum interlayer spacing at which Na+can be reversibly intercalated between carbon layers is 3.7 Å.
Carbon based hollow nanospheres were prepared by hydrothermal method. After 100 cycles at a current density of 50mA/g, the reversible capacity still reached 200mA · h/g, demonstrating excellent cycling performance. Low porosity hard carbon was prepared using sucrose, with a reversible capacity of 335mA · h/g at a current density of 40mA/g. After 500 cycles, the capacity was still maintained at 300mA · h/g.
Researchers believe that porosity and specific surface area are inversely proportional to reversible capacity, and increasing porosity and specific surface area can lead to a sharp decrease in reversible capacity. By doping Graphite oxide into sucrose precursor, the specific surface area of sucrose hard carbon was effectively reduced from 137.2m2/g to 5.4m2/g. When the current density was 20mA/g, the first coulomb efficiency was increased from 74% to 83%, and the reversible capacity was 280mA · h/g. After 200 cycles, the capacity remained 95%. Because this doping can effectively reduce the foam of sucrose coking reaction, the specific surface area of carbonized materials can be significantly reduced. Synthesize nitrogen doped porous carbon (NPC), with a current density of 50mA/g and a reversible capacity of 144.7mA · h/g after 200 cycles; When the current density is 400mA/g, the reversible capacity is 117.6mA · h/g, demonstrating good cycling and rate performance. It is believed that the good performance is attributed to the doping of nitrogen atoms and the mesoporous structure of the material. Synthesis of nitrogen doped nanofibers( CNF@NPC )When the current density is 100mA/g, after 100 cycles, the reversible capacity is 240mA · h/g; At a current density of 1000mA/g, the reversible capacity can still reach 146.5mA · h/g, exhibiting excellent rate performance. A pyrolytic carbon material was obtained by optimizing the mixing ratio of asphalt and phenolic resin and the pyrolysis temperature. The first coulomb efficiency reached 88%, the reversible capacity was 284mA · h/g, and it had excellent cycle stability, providing a method for preparing low-cost negative electrode materials for Sodium-ion battery.
In summary, it can be seen that in the past decade, researchers have studied many non graphite carbon negative electrode materials with large interlayer spacing and disordered structure, which are conducive to Na+de intercalation. These studies have demonstrated the feasibility of non graphite carbon materials, and have achieved significant breakthroughs in reversible capacity, cycling performance, and magnification performance.




 Phone:+86 13861313805
Phone:+86 13861313805
 E-mail:louis.han@e-tygroup.com
E-mail:louis.han@e-tygroup.com
 Add:Room 2809, Building Ⅱ, The Gate of the Orient, No. 199 Xinggang Street, Suzhou Industrial Park, Suzhou, China.
Add:Room 2809, Building Ⅱ, The Gate of the Orient, No. 199 Xinggang Street, Suzhou Industrial Park, Suzhou, China.
