"Hard carbon" high-quality sodium lithium-ion batteries usher in a new trend
Sodium-ion battery has always been considered as an industrializable product that can replace part of the industrial space of lithium ion battery. In 2022, a large number of domestic institutions have invested in the field of Sodium-ion battery. This year is generally considered to be the first year of Sodium-ion battery industrialization in China. However, due to factors such as unstable precursor sources and low compaction density, it has been delayed in truly opening up the space for industrialization.
Amorphous carbon can be divided into soft carbon and hard carbon according to the degree of graphitization difficulty. Hard carbon materials have significant advantages in Sodium-ion battery
Hard carbon is a carbon material that is difficult to convert into graphite after high-temperature treatment above 2800 ℃. It is difficult to eliminate the disordered structure of hard carbon at high temperatures, and can also be called difficult graphitized carbon. Although the electronic conductivity of soft carbon materials is high, their specific capacity is still relatively low, limiting the widespread application of soft carbon in sodium electricity. Unlike soft carbon sodium storage methods, the capacity and voltage curves of hard carbon materials exhibit a coexistence of slopes and platforms. As a sodium ion negative electrode material, hard carbon has a reversible specific capacity of up to 300mAh/g. The hard carbon negative electrode has a low sodium storage potential, which is beneficial for improving the voltage and energy density of the entire battery. At the same time, it has a highly disordered structure and exhibits good cycling performance.
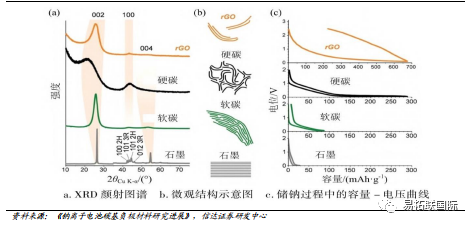
Biomass hard carbon route is expected to be the first to achieve industrialization
Hard carbon negative electrode precursors mainly include biomass, resin, coal based and asphalt, and cost, performance, and stability of raw material supply are the main considerations for industrialization. Resins are mainly chemical polymers, with high raw material prices, but high raw material purity, low production difficulty, and good product consistency. The supply of asphalt and coal raw materials is sufficient, and the price is cheap. However, due to the immature process, the product performance is inferior to that of biomass. Biomass has a wide range of sources, low prices, and high hard carbon capacity, which is expected to be the first to benefit from the industrialization process of sodium electricity 0-1. At present, mainstream negative electrode companies have reserves of biomass hard carbon products. With technological upgrading and accelerated mass production, the cost of hard carbon is expected to further decrease.
Lithium negative electrodes are not compatible with sodium batteries, and the development of sodium batteries requires the development of new negative electrode material systems
Anode material is one of the key materials in the development and application of Sodium-ion battery technology. Since the Ionic radius of sodium is far larger than the Ionic radius of lithium, the structure of sodium ions will change greatly during the process of removal and insertion, resulting in the reduction of reversible capacity. In addition, due to thermodynamic reasons, the graphite anode has poor sodium storage performance, so the research and development of negative electrode materials for Sodium-ion battery is facing great challenges. Similar to lithium ion battery, Sodium-ion battery is also a "rocking chair" charging and discharging principle. The two negative electrodes have similarities. Looking for anode materials with superior performance and application prospects for Sodium-ion battery should meet seven major factors:
1) High sodium storage capacity and electrochemical reversible performance.
2) The oxidation Reduction potential should be as close to the metal sodium potential as possible.
3) During the process of de embedding, there is minimal structural change and good cyclic stability.
4) Good compatibility with electrolyte solutions and no side reactions with the components in the electrolyte.
5) High ion mobility and electronic conductivity.
6) Good chemical and thermodynamic stability.
7) The preparation process is simple and controllable, the raw materials are easy to obtain, and it is environmentally friendly.
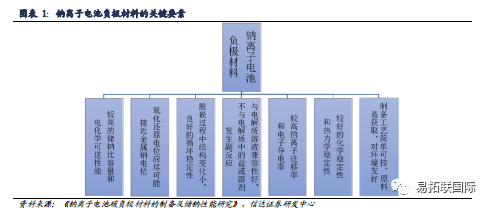
Carbon based negative electrode materials are currently the mainstream negative electrode materials for sodium batteries. In the process of charging/discharging of Sodium-ion battery, it is necessary to rely on the negative electrode material of Sodium-ion battery as the main carrier for sodium ion storage in the sodium battery to realize the insertion/removal of sodium ions. The selection of different negative electrode materials directly affects the electrochemical performance of the sodium battery. At present, carbon based materials, alloy materials, metal/sulfide materials, Titanate materials, metal oxide materials and other organic compound materials are the main anode materials for Sodium-ion battery. Among them, carbon based sodium storage anode materials (graphite, Graphene, hard carbon, soft carbon, etc.) have outstanding advantages such as low sodium insertion platform, high capacity, long cycle life, simple preparation, environmental friendly, non-toxic, etc., while other materials are limited by high costs and difficult to obtain compared with carbon materials, and carbon materials are still the main anode materials for Sodium-ion battery.
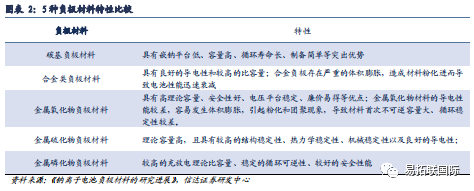
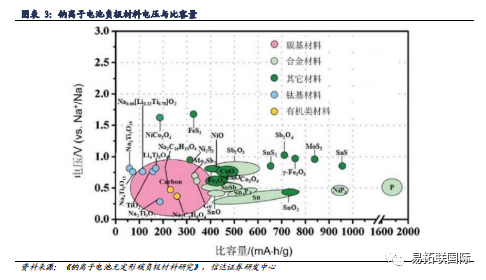
Carbon based materials mainly include graphite, soft carbon, and hard carbon, and the development prospects of hard carbon negative electrode sodium electricity are bright
The characteristics of graphite and sodium ions determine that graphite negative electrodes are not suitable for sodium batteries.
1) In terms of physical structure, graphite includes modified natural graphite and artificial graphite, which is a carbon material with a regular layered structure and is currently mainly used as the negative electrode for lithium batteries. The radius of sodium ions is 0.102nm, which is much larger than the radius of lithium ions (0.069nm). The interlayer spacing between graphite is small (0.334nm), making it difficult for sodium ions to effectively intercalate in graphite. Using graphite as the negative electrode material for sodium batteries has a specific sodium storage capacity of only 35mAh/g.
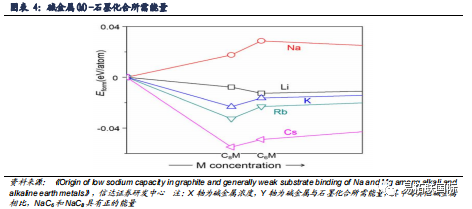
2) Thermodynamically, according to the literature "Origin of low sodium capacity in graphite and generally weak substrate binding of Na and Mg among alkali and alkaline earth metals", the energy required for the formation of compounds between alkali metals (M) and graphite is in descending order: Na>Li>K>Rb>Cs (Figure 4). Graphite compounds with high sodium content exhibit thermodynamic instability. Theoretical calculations indicate that the low sodium storage capacity of graphite is attributed to thermodynamic factors. The weak interaction between sodium ions and graphite layers makes it difficult for sodium ions to form stable intercalation compounds with graphite, which is the reason for the low sodium storage capacity of graphite.
3) Inappropriate electrolyte: graphite cannot be effectively intercalated in traditional carbonate electrolyte, but after sodium ions and ether solvents form Solvation molecules, it can be effectively intercalated into the graphite layer, and has a reversible capacity of about 100mAh/g by forming a first-order ternary intercalation compound. However, ether based electrolytes are prone to decomposition under high voltage, and their sodium storage capacity is still low, consuming solvents. Additionally, their sodium storage potential is high, resulting in significant volume changes, reducing the energy density and cycle life of sodium batteries.
The hard carbon negative electrode is on the eve of the outbreak from zero to one and is fully promoting localization. At present, Beisige and Beiterui have hard carbon negative electrode production capacity and are fully promoting the localization of biomass based hard carbon; Shanshan Co., Ltd., Zhongke Electric, Xiangfenghua and other leading companies in the field of artificial graphite negative electrodes are respectively laying out routes for biomass based, fossil fuel based, and synthetic polymer based hard carbon negative electrodes; Newly entered enterprises such as Yuanli Group and Shengquan Group have also laid out biomass based hard carbon materials, among which Shengquan Group mainly relies on the advantage of biomass production from straw sources.
With the maturity of the sodium battery material system and the scaling of production capacity, with excellent economy and safety, the global demand for sodium batteries is expected to exceed 120GWh in 2026, corresponding to a market space exceeding 60 billion yuan; It is expected that the demand for hard carbon negative electrodes will reach 162300 tons in 2026, and the corresponding market space is expected to reach 7.3 billion yuan.




 Phone:+86 13861313805
Phone:+86 13861313805
 E-mail:louis.han@e-tygroup.com
E-mail:louis.han@e-tygroup.com
 Add:Room 2809, Building Ⅱ, The Gate of the Orient, No. 199 Xinggang Street, Suzhou Industrial Park, Suzhou, China.
Add:Room 2809, Building Ⅱ, The Gate of the Orient, No. 199 Xinggang Street, Suzhou Industrial Park, Suzhou, China.
