Carbon electrode material
Carbon is the earliest electrode material used to manufacture supercapacitors, and it has been developed for half a century since Beck published the relevant patent in 1954.
Carbon electrode capacitors mainly utilize the double layer energy stored at the electrode/electrolyte interface, and their specific surface area is an important factor determining the capacitor capacity. Many different types of carbon materials have been proven to be used for the production of polarized electrodes for supercapacitors, such as activated carbon (AC), activated carbon fibers (ACF), carbon aerosols (CAGs), and carbon nanotubes (CNTs).

At present, some people use PVdF HFP gel electrolyte as adhesive and activated carbon powder to prepare carbon electrode, with specific surface area of 2500 ㎡/g, specific capacity of 123 F/g, and cycle life of 104 times. Japanese scholar Hiroyuki et al. prepared high-density activated carbon fibers (HD-ACF) using hot pressing method, with a density of 0.2g/cm ³、 0.8g/cm ³。 In addition, some new carbon materials, such as carbon nanotubes and carbon nanotubes, have made some progress in the application of electrochemical capacitors. However, the preparation of carbon nanotubes is difficult and the production cost is high, which is a major problem limiting their application.
Activated carbon (AC)
Activated carbon is the most commonly used electrode material for supercapacitors. It has the characteristics of rich raw materials, low price, good formability, high electrochemical stability, and mature technology. The specific capacitance of activated carbon Supercapacitor is related to its specific surface area and pore size distribution. It is generally believed that the larger the specific surface area of activated carbon, the higher its specific capacity. Therefore, high specific capacity can usually be obtained by using electrode materials with large specific surface areas. At present, commonly used activation methods to increase its specific surface area include chemical activation and physical activation.
Chemical activation is the use of certain acids (such as HNO Å) or bases (such as KOH) for chemical corrosion to increase the specific surface area and surface functional groups of materials, or the use of surfactants (such as sodium oleate) for chemical modification of materials to improve the wettability of the electrolyte in the material, thereby increasing the specific capacitance. After activating AC with KOH at 850 ℃, LotaG et al. significantly improved the specific surface area, specific capacitance, and energy density of AC. The specific capacitance reached 200F/g in 1mol/L H ₂ SO ₄ and 6mol/L KOH aqueous electrolytes, and the specific capacitance reached 150F/g in 1mol/L (C ₂ H Å) NBF ₄ electrolytes, and the charge discharge performance was significantly improved.
Physical activation refers to the use of activation agents such as water vapor or carbon dioxide in the precursor to promote the formation of surface pore structures, increase specific surface area, and thus form pores suitable for adsorption, thereby increasing the specific capacitance of the material. Alar Janes et al. activated commercial nano porous carbon using water vapor at temperatures ranging from 950 to 1050 ℃, resulting in a specific surface area of 2240 ㎡/g.

At present, activated carbon materials with a specific surface area exceeding 3000 ㎡/g have been prepared, but their true utilization rate for supercapacitor electrodes is only about 30%. Therefore, the commonly used AC specific surface area is about 1500 ㎡/g, generally not exceeding 2000 ㎡/g, and its high specific capacity can reach 280F/g (aqueous electrolyte) and 120F/g (non aqueous electrolyte).
In the early days, practical polarization electrodes were made of activated carbon powder. For example, paste electrodes were made by mixing activated carbon powder and electrolyte. Later, active carbon powder was bonded with polytetrafluoroethylene or Carboxymethyl cellulose as binder, and the electrodes were made into round sheets and solid electrodes of activated carbon/carbon complex.
With the development of science and technology, new methods have emerged for the preparation of electrode materials from activated carbon. Osaka et al. used Polyvinylidene fluoride Hexafluoropropylene (PVdF HFP) gel electrolyte as a binder and activated carbon powder to prepare activated carbon electrode (activated carbon/PVdF HFP, mass ratio 7/3), with a specific surface area of 2500 ㎡/g, a specific capacity of 123 F/g, and a cycle life of 104. N. L. Wu et al. added conductive carbon black with a small specific surface area (220 ㎡/g) to a non-conductive activated carbon with a large specific surface area (1420 ㎡/g). When the carbon black reached 25% (mass fraction), in a 1M KOH aqueous solution, and at a voltage scanning rate of 20mV/s, the larger specific capacitance was 108F/g. The reason for the differences in the electrochemical performance of carbon materials is related to the subtle differences in raw materials and preparation processes. More in-depth and detailed research is needed to meet the urgent need for Supercapacitor in China.
Activated Carbon Fiber (ACF)
Activated carbon fiber is a new type of high-efficiency adsorption material that began to develop with the development of industry in the 1970s. It is a high-performance activated adsorption material and environmental engineering material with superior performance than activated carbon. Activated carbon contains large open pores and has excellent adsorption, drug resistance, hydrophobicity, electronic supply, and large specific surface area characteristics.
The preparation of activated carbon fibers forms the precursor of activated carbon, which has a high specific surface area after stabilization and carbonization treatment. Bin Xue et al carbonized Polyacrylonitrile with CO2, optimized the carbonization temperature, and obtained a large specific surface area and a suitable pore size distribution of activated carbon fiber network structure material at 600 ℃.
Due to the strength and formability of the fibers themselves, which are lacking in activated carbon, and the advantage of activated carbon fibers is that they can provide a good reaction path and control pore size and distribution through the process. Activated carbon fibers used as electrode materials will have a more concentrated pore distribution and smaller pores (which can be used to store charges), and have strong adsorption and desorption ability. If properly treated, a considerable specific surface area (mostly micropores) will be obtained. Therefore, it is suitable to use as an electrode material for supercapacitors. However, due to the high cost of activated carbon fibers, their industrial production is limited.

SEM images of activated carbon fibers from coniferous wood
Carbon Aerogel (CAGs)
Carbon Aerogel is a new type of lightweight nano porous amorphous carbon material. It is characterized by stable performance, high charging and discharging efficiency, and is suitable for high current charging and discharging. At the same time, adding carbon Aerogel to the high specific surface active carbon can improve the specific capacitance of the electrode. When the carbon Aerogel content is 15%, the specific capacitance of the electrode is high. Due to its high specific surface area and wide range of density changes, it has special properties in electrical, thermal, optical, and other fields, and has broad application prospects. Especially, its large specific surface area and high conductivity make it an ideal electrode material for supercapacitors.
The preparation of carbon Aerogel is relatively complex, and generally can be divided into three steps: organic gel formation, supercritical drying and carbonization. The organic gel can be formed into a gel with three-dimensional spatial network structure; Supercritical drying can maintain the texture of gel and remove the solvent in the pores; Carbonization strengthened the texture of gel; It increases the mechanical properties and maintains the texture of organic gel. The carbon gel synthesized by Meng et al. through sol/gel has high mesopore content and large pore volume, with an average pore size of 81.89nm. The specific capacity of the prepared electrode is 121F/g, and the charge discharge efficiency is 95%. Although carbon gel has excellent performance, its commercialization process is restricted by the long preparation time, expensive and complex supercritical drying equipment.
At present, the research direction of carbon gel Supercapacitor is to control reaction conditions, prepare carbon gel with specific pore structure, and find other drying methods that can replace supercritical drying.
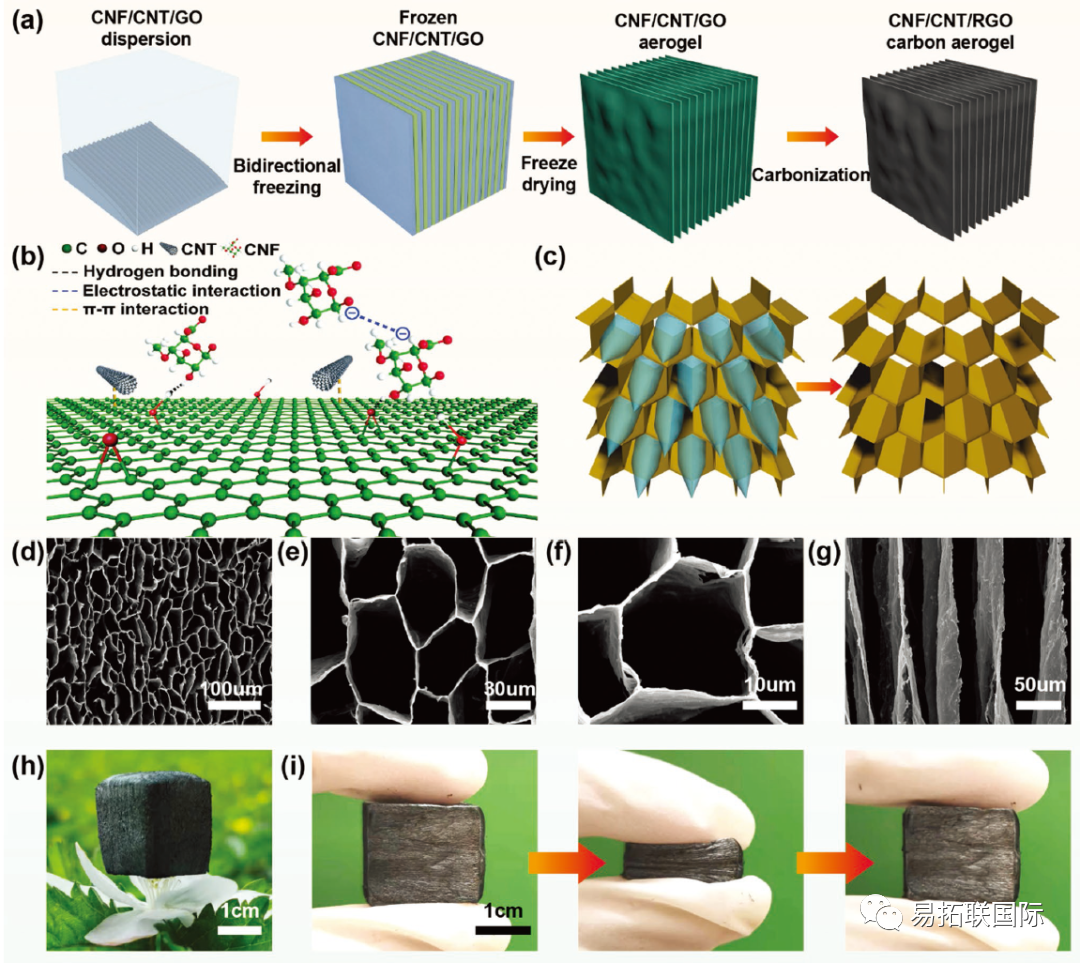
Fig. 1. a) Schematic diagram of CNF/CNT/RGO carbon Aerogel preparation. b) Schematic diagram of the interaction between CNF, CNT, and RGO. c) Schematic diagram of ice crystal growth mechanism. D-f) Top view and side view SEM images of CNF/CNT/RGO-3 carbon Aerogel. h) The optical image shows an ultra light CNF/CNT/RGO Aerogel standing on a flower. i) Compression recovery process of carbon Aerogel.
Carbon nanotubes (CNTs)
Carbon nanotubes are seamless hollow tubes formed by curling single-layer or multi-layer carbon graphite sheets, with a diameter of 14-100nm. Therefore, they have advantages such as large specific surface area, high crystallinity, good conductivity, concentrated micropores, and controllable size. They are an ideal electrode material that can fully utilize the principle of quasi capacitive energy storage.
Another important feature of carbon nanotubes is their unique hollow cavity structure (with pore sizes mostly ranging from 2-50nm), which exhibits an interwoven network distribution, and the size of the micropores can be controlled through synthesis processes. Therefore, when carbon nanotubes are used as the electrodes of Supercapacitor, they have a high utilization ratio of specific surface area. According to the literature currently reported, there are generally two methods for using carbon nanotubes as electrode materials: one is the method of adding adhesive to form the electrode; The other is directly filtered and heated. Capacitor electrode material made by direct Thermoforming method, with unit specific surface area of 430 ㎡/g; Using 38wt% sulfuric acid as the electrolyte and polymer as the interlayer, the higher capacity can reach 113F/g, and at 0.1Hz, the capacity can reach 108F/g. The capacitor electrode material formed by bonding is also used, with 38wt% sulfuric acid as the electrolyte, phenolic resin as the adhesive, glass fiber as the interlayer, and graphite sheet as the collector. The specific capacity can reach 15.25F/cm ², After further improvement, the specific capacity of the capacitor can reach 107F/cm ², That is 600F/g.
Jiang Qi and others from Chengdu Institute of Organic Chemistry, Chinese Academy of Sciences conducted a preliminary study on the electrochemical supercapacitor performance of carbon nanotubes, and believed that multi walled nanotubes with small diameter, length and low graphitization degree of carbon nanotubes are more suitable for the electrode materials of Supercapacitor. At present, the industrial production technology of carbon nanotubes is not yet mature and the price is very high. Its application in capacitors is also in the research stage, and there is still a long distance from practical application.
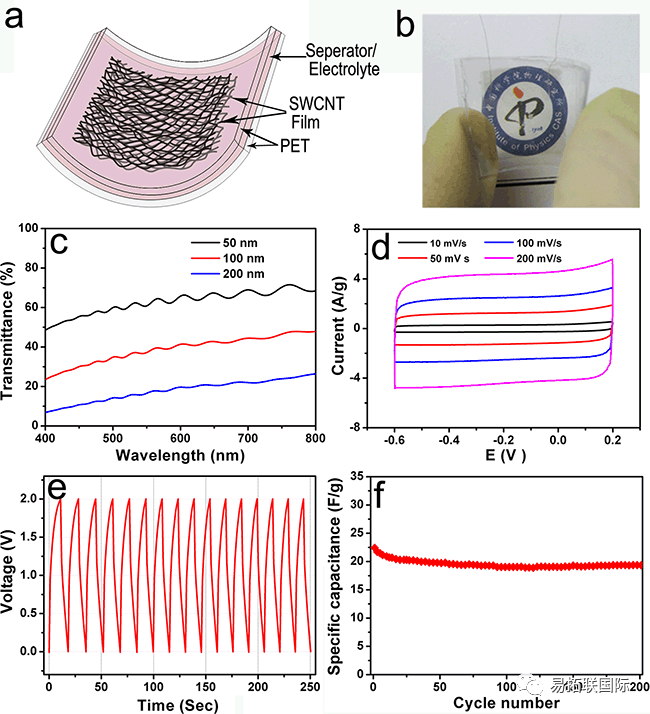
(a) Schematic diagram of transparent supercapacitor structure and (b) optical photos; (c) The transmittance of supercapacitors assembled with carbon nanotube thin films of different thicknesses, and the CV curves of flexible transparent carbon nanotube thin film supercapacitors (50 nm) at different scanning rates; (e) Constant current charging and discharging curve; (f) The variation of specific capacitance with the number of charges and discharges.
Supercapacitor activated carbon is a new type of highly adsorbed activated carbon, which is mainly used in supercapacitors (also known as Supercapacitor, electrochemical capacitors). It has a large specific surface area, good electrochemical performance, and high capacity. Supercapacitors are a type of passive device that has only been mass-produced in recent years, located between batteries and ordinary capacitors. They have the characteristics of high current fast charging and discharging of capacitors, as well as the energy storage characteristics of batteries. They have a long reusable life and use electrons between moving conductors (rather than relying on chemical reactions) to release current during discharge, thereby providing power for the equipment. Supercapacitors have an extremely long working life and fast charging and discharging characteristics, and are also widely used in various fields such as electric vehicles, hybrid vehicles, electric tools, electric toys, railway systems, and power systems.




 Phone:+86 13861313805
Phone:+86 13861313805
 E-mail:louis.han@e-tygroup.com
E-mail:louis.han@e-tygroup.com
 Add:Room 2809, Building Ⅱ, The Gate of the Orient, No. 199 Xinggang Street, Suzhou Industrial Park, Suzhou, China.
Add:Room 2809, Building Ⅱ, The Gate of the Orient, No. 199 Xinggang Street, Suzhou Industrial Park, Suzhou, China.
