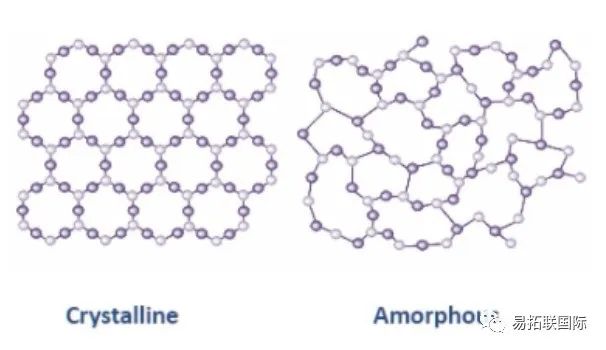
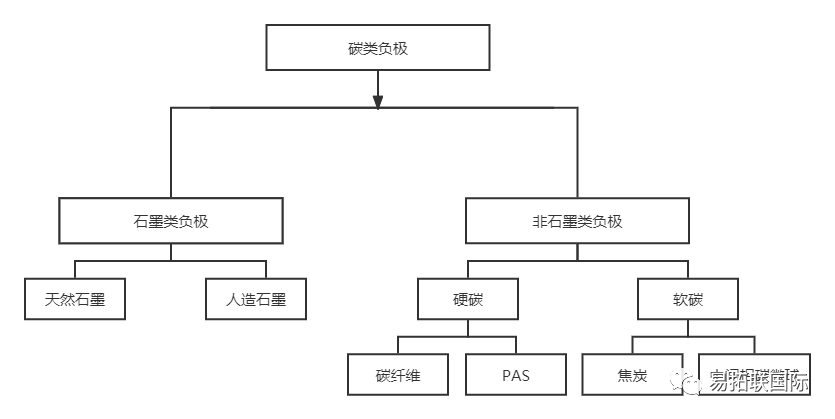
Graphite mainly consists of the hexagonal structure (2H or phase) of ABAB stacking and the diamond structure (3R or phase) of ABCABC stacking. The two phases of graphite can be converted to each other, and mechanical treatment and other processes can lead to an increase in the proportion of phase composition in the graphite. Annealing treatment at high temperatures will generate more thermodynamic stable phases. Graphite has become the most common anode material for commercial lithium-ion batteries due to its long range ordered stacking structure, good conductivity, high specific capacity and good cycling performance. Its raw materials are mainly asphalt, Petroleum coke and natural graphite, and the layer spacing is about 0.335 to 0.34 nm. Amorphous carbon mainly includes hard carbon and soft carbon, which are usually composed of randomly distributed graphitized microstructures, twisted Graphene nanosheets and pores between the above-mentioned microstructures, lacking an ordered stacking structure. Soft carbon, also known as easily graphitized carbon, transforms into graphite at high temperatures above 2800 ° C. The crystal is similar to graphite but has a lower degree of order. The short range ordered graphitized microcrystalline structure is conducive to intercalation and sodium storage. Hard carbon is non graphitized carbon. It is difficult to graphitize even when heated to 2800 ° C. Its structure is highly disordered and its oxidation Reduction potential is low. It is considered to be an ideal anode material for Sodium-ion battery.
Although graphite itself has a good specific lithium storage capacity (372mAh/g), it has also played an important role in the field of lithium ion batteries. However, due to the large sodium Ionic radius, it has hindered the insertion and removal of sodium ions during the charge and discharge process, making graphite unable to become a suitable negative electrode material for Sodium-ion battery. People have also tried various methods to improve the sodium storage performance of graphite, but the results are not satisfactory at present.
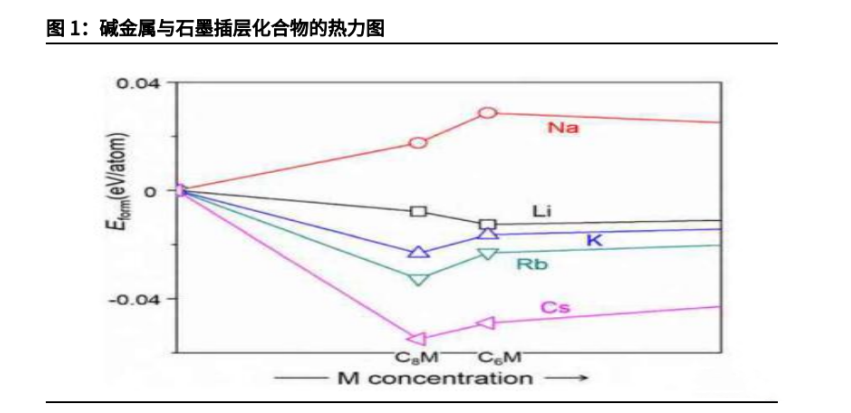
The first method is to expand the spacing of graphite layers to improve their sodium storage performance. Research has found that expanded graphite with a interlayer spacing of 0.43nm has a specific capacity of 184mAh/g and a capacity retention rate of 73.92% after 2000 cycles at 5C magnification. However, X-ray diffraction spectra show that the ordered structure in the expanded graphite is disrupted, essentially due to the amorphous nature of the expanded graphite. It can enable more Na+to be reversible disembedded in the graphite, but this kind of reduced Graphite oxide still has the problem of low ICE (mainly because of the unavoidable electrolyte decomposition and the non Reversible reaction between Na+and oxygen containing functional groups on the reduced Graphite oxide sheet leading to the formation of thick SEI film), and the storage mechanism of Na+in the reduced Graphite oxide is still unclear.
Another method is to improve the sodium storage performance of graphite by adjusting the electrolyte. In the test, it was found that the stable sodium graphite intercalation compound could not be formed in the carbonate electrolyte, which limited the sodium storage performance of graphite. In the ether based electrolyte, although the Solvation sodium ion can indirectly store sodium by co intercalation to form a Na solvent molecule graphite ternary intercalation compound, it has low specific capacity and high sodium storage potential The problems of poor oxidation resistance and stability of ether based solvents, which are easy to react with the positive electrode, are still difficult to overcome in the practical application of graphite as the negative electrode material of Sodium-ion battery.
Although graphite itself has a good specific lithium storage capacity (372mAh/g), it has also played an important role in the field of lithium ion batteries. However, due to the large sodium Ionic radius, it has hindered the insertion and removal of sodium ions during the charge and discharge process, making graphite unable to become a suitable negative electrode material for Sodium-ion battery. People have also tried various methods to improve the sodium storage performance of graphite, but the results are not satisfactory at present.
The first method is to expand the spacing of graphite layers to improve their sodium storage performance. Research has found that expanded graphite with a interlayer spacing of 0.43nm has a specific capacity of 184mAh/g and a capacity retention rate of 73.92% after 2000 cycles at 5C magnification. However, X-ray diffraction spectra show that the ordered structure in the expanded graphite is disrupted, essentially due to the amorphous nature of the expanded graphite. It can enable more Na+to be reversible disembedded in the graphite, but this kind of reduced Graphite oxide still has the problem of low ICE (mainly because of the unavoidable electrolyte decomposition and the non Reversible reaction between Na+and oxygen containing functional groups on the reduced Graphite oxide sheet leading to the formation of thick SEI film), and the storage mechanism of Na+in the reduced Graphite oxide is still unclear.
Another method is to improve the sodium storage performance of graphite by adjusting the electrolyte. In the test, it was found that the stable sodium graphite intercalation compound could not be formed in the carbonate electrolyte, which limited the sodium storage performance of graphite. In the ether based electrolyte, although the Solvation sodium ion can indirectly store sodium by co intercalation to form a Na solvent molecule graphite ternary intercalation compound, it has low specific capacity and high sodium storage potential The problems of poor oxidation resistance and stability of ether based solvents, which are easy to react with the positive electrode, are still difficult to overcome in the practical application of graphite as the negative electrode material of Sodium-ion battery.
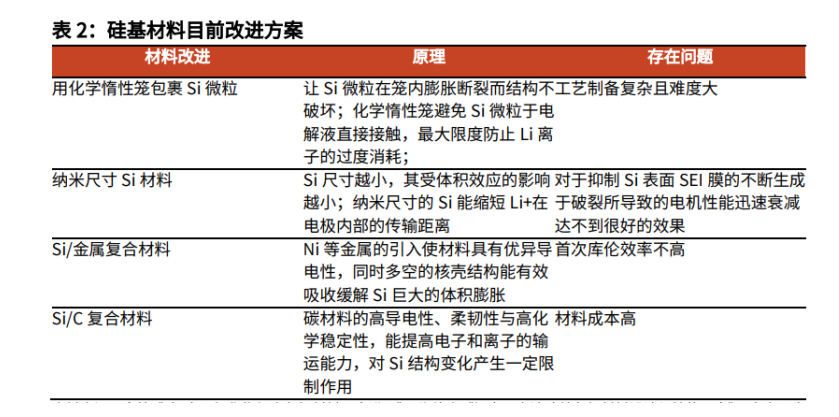
3.1. Silicon based negative electrode materials
Although graphite itself has a good specific lithium storage capacity (372mAh/g), it has also played an important role in the field of lithium ion batteries. However, due to the large sodium Ionic radius, it has hindered the insertion and removal of sodium ions during the charge and discharge process, making graphite unable to become a suitable negative electrode material for Sodium-ion battery. People have also tried various methods to improve the sodium storage performance of graphite, but the results are not satisfactory at present.
The first method is to expand the spacing of graphite layers to improve their sodium storage performance. Research has found that expanded graphite with a interlayer spacing of 0.43nm has a specific capacity of 184mAh/g and a capacity retention rate of 73.92% after 2000 cycles at 5C magnification. However, X-ray diffraction spectra show that the ordered structure in the expanded graphite is disrupted, essentially due to the amorphous nature of the expanded graphite. It can enable more Na+to be reversible disembedded in the graphite, but this kind of reduced Graphite oxide still has the problem of low ICE (mainly because of the unavoidable electrolyte decomposition and the non Reversible reaction between Na+and oxygen containing functional groups on the reduced Graphite oxide sheet leading to the formation of thick SEI film), and the storage mechanism of Na+in the reduced Graphite oxide is still unclear.
Another method is to improve the sodium storage performance of graphite by adjusting the electrolyte. In the test, it was found that the stable sodium graphite intercalation compound could not be formed in the carbonate electrolyte, which limited the sodium storage performance of graphite. In the ether based electrolyte, although the Solvation sodium ion can indirectly store sodium by co intercalation to form a Na solvent molecule graphite ternary intercalation compound, it has low specific capacity and high sodium storage potential The problems of poor oxidation resistance and stability of ether based solvents, which are easy to react with the positive electrode, are still difficult to overcome in the practical application of graphite as the negative electrode material of Sodium-ion battery.
3.3 Tin based negative electrode material
Tin based negative electrode materials have also attracted widespread attention from scholars and entrepreneurs. Its advantages lie in: rich resources (by 2019, China's Proven reserves were 2.16 million tons, accounting for 39.27% of the world's total, ranking first in the world); High theoretical capacity (Li22Sn5 produces a total theoretical capacity of approximately 994mAh/g, while Na15Sn4 produces a theoretical capacity of 847mAh/g); The lithium intercalation potential is higher than the lithium precipitation potential, which can avoid lithium deposition at high magnification; The packing density is high (75.46mol/L, which is close to the packing density of 73.36mol/L for lithium). The disadvantage is that during the cycle, the volume expansion rate of Sn reaches 259% (lithium ion battery) and 423% (Sodium-ion battery) respectively, which seriously affects the cycle performance. The current improvement measures include: (1) reducing the particle size of Sn to the nanoscale: there may be uneven particle size and agglomeration phenomena, and the advantages of high capacity of Sn cannot be demonstrated; (2) Tin based alloy materials: The negative electrode materials of lithium-ion batteries have entered a stable development stage - most of the tin based alloys and heterogeneous components are only physically composite, and weak interfacial forces are not conducive to the development of composite effects; Anode materials for Sodium-ion battery are under exploration; (3) Tin based/carbon composite material: Sn alloy particles wrapped in an elastic carbon shell can stably complete the lithium insertion and removal process.
The information provided in this article is for general guidance and information purposes only, and the content of this article should not be considered as investment, business, legal or tax advice under any circumstances.




 Phone:+86 13861313805
Phone:+86 13861313805
 E-mail:louis.han@e-tygroup.com
E-mail:louis.han@e-tygroup.com
 Add:Room 2809, Building Ⅱ, The Gate of the Orient, No. 199 Xinggang Street, Suzhou Industrial Park, Suzhou, China.
Add:Room 2809, Building Ⅱ, The Gate of the Orient, No. 199 Xinggang Street, Suzhou Industrial Park, Suzhou, China.
