
Hard carbon vs soft carbon
According to the difficulty of graphitization, Amorphous carbon materials can be divided into hard carbon and soft carbon. Soft carbon usually refers to carbon materials that can be graphitized after high-temperature treatment (above 2800 ℃), and the disordered structure is easily eliminated, also known as easily graphitized carbon. Hard carbon usually refers to carbon that is difficult to fully graphitize after high-temperature treatment (above 2800 ℃), and its disordered structure is difficult to eliminate at high temperatures, also known as difficult to graphitize carbon. Under moderate and low temperature (1000-1600 ℃) treatment, there is no obvious boundary between soft carbon and hard carbon in structure, which can be uniformly called Amorphous carbon.
Although soft carbon materials have high capacity values, their fast decay rate poses obstacles to practical applications; Hard carbon materials are easier to prepare and have a longer cycle life, which has been partially applied in practical applications. Compared with soft carbon, hard carbon has more disordered structure, higher defect concentration, higher heteroatom content, larger distance between graphite layers, and more closed pore structure. This is beneficial for providing more storage points and diffusion pathways for Na+ions. But the economy of hard carbon is slightly worse than that of soft carbon. Among Sodium-ion battery, hard carbon is the mainstream of current application with its advantages. In addition, the characteristics of low cost, sustainability, and simpler preparation also provide more possibilities for the commercialization of hard carbon materials.
Soft carbon and hard carbon mainly depend on the properties of the precursor. During the carbonization process, the precursor can appear in a fusion state over a wide temperature range, which is a necessary condition for the final graphitization of carbon (coke). This fusion state allows for the rearrangement of carbon layers, forming a long-range ordered layered structure. The gas generated by thermal decomposition is easy to escape, while the carbon content and density of the residue increase. Amorphous carbon is usually produced by pyrolysis of organic precursors at 500-1500 ℃. The final product of pyrolysis, whether it is hard carbon or soft carbon, mainly depends on the properties of the precursor.
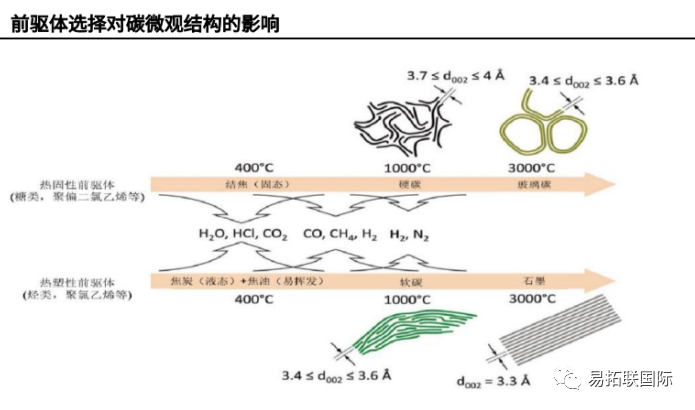
Precursors are mainly divided into biomass based, polymer based, resin based, and coal based carbon materials. Biomass precursors mainly refer to the roots, stems and leaves of plants (such as Banana peel, peat moss, peanut shell, leaves, apple peel, pomelo peel, poplar and cotton). Polymers usually refer to chemical products derived from biomass extraction, mainly including glucose, sucrose, starch, cellulose, and lignin, which are precursors of carbohydrates. Resin precursors mainly include phenolic resin, Polyaniline and Polyacrylonitrile.
Biomass, resin and polymer precursors are mainly used to produce hard carbon. Precursors for preparing soft carbon materials mainly include petrochemical raw materials and their downstream products, such as coal, asphalt, Petroleum coke, etc., but soft carbon materials directly carbonized show low reversible capacity in Sodium-ion battery.
The core Technology roadmap of hard carbon preparation includes raw material selection and pretreatment, cross-linking and curing, carbonization and purification. There are also technological differences in the preparation process of hard carbon negative electrode materials for different types of precursors. The temperature control of the intermediate steps, gas atmosphere, and heating time affect the pore size, purity, oxygen content, and specific surface area of the negative electrode material. It also indirectly affects factors such as the initial efficiency, energy density, and safety of the battery.
The molecular structure of organic polymer precursors is relatively simple and controllable, and can design relevant molecular structures according to needs, so it is an excellent precursor for preparing carbon materials, and has attracted extensive attention. Organic polymer is prepared by catalytic polymerization of organic small molecule monomers through catalysts. Due to its advantages of obtaining regular morphology of hard carbon structures and simple synthesis process, it has high research value for the large-scale production and application of hard carbon materials in the future. Phenolic resin material (RF) is currently a well researched organic polymer, which has received widespread attention due to its high carbon residue rate and excellent structural stability after forming hard carbon. Research has found that the time required for polymerization depends on the solvent used, while the yield depends on the degree of crosslinking, which can be controlled by adjusting the thermal polymerization temperature. The structure of hard carbon mainly varies with the carbonization temperature, and as the temperature increases, the interlayer spacing and disorder decrease, thereby affecting the sodium storage performance of the material. And regulating the formation of hard carbon pore structure is beneficial for improving electrochemical performance. A study by the Kamiyama team abroad found that as the heat treatment temperature increases, the interlayer distance decreases, the specific surface area decreases, and the internal pores increase. The unique macroporous phenolic resin increases the total volume of internal pores, thereby increasing the sodium ion capacity.

There are a variety of biomass precursors with the characteristics of sustainable use and low cost. Usually contains a large amount of C and some O, H, and even some other heteroatoms, such as N, S, P, etc. Biomass is a good choice for producing renewable and sustainable precursors of low-cost and high-performance hard carbon anode materials. The methods for converting biomass into hard carbon are simple, such as direct carbonization, hydrothermal carbonization (HTC), physical or chemical activation, etc. Biomass such as Banana peel, peat moss, rice husk, cotton, glucose, protein and cellulose nanocrystals have been used as anode materials for Sodium-ion battery, showing good electrochemical performance. However, on the other hand, biomass from nature usually contains some impurities, which need to be removed before it is applied to Sodium-ion battery. Most reported biomass derived hard carbon anodes can provide a reversible capacity of up to 300mAh/g, while ICE (first week coulomb) is below 85%, which still cannot compete with graphite anodes in commercial lithium batteries. Despite the sustainability and richness of biomass, the overall cost is still higher than that of graphite and soft carbon.
Asphalt, as a low-cost petrochemical byproduct, is currently widely used due to its low cost and high carbon content. However, asphalt based materials are prone to forming ordered structures during high-temperature cracking, resulting in a very low storage capacity of less than 100mAh/g. At present, the Chinese Academy of Sciences has improved the sodium storage capacity to 300mAh/g by compounding asphalt as soft carbon precursor and resin as hard carbon precursor.
Anthracite based precursors have high cost performance ratio and broad market application prospects. The Institute of Physics of the Chinese Academy of Sciences uses Anthracite as the precursor to obtain a carbon anode material with excellent sodium storage performance through simple crushing and one-step carbonization. The soft carbon material obtained by cracking Anthracite still has a high degree of disorder below 1600 ° C, with a carbon production rate of 90%, a sodium storage capacity of 220mAh/g, excellent cycle stability, and better performance than the soft carbon material from asphalt.
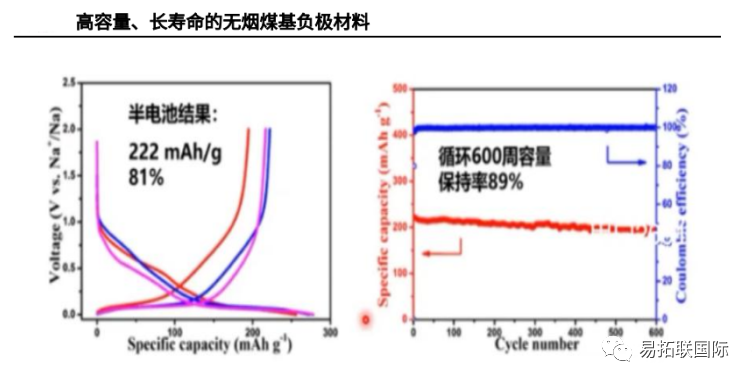
Zhongke Haina also investigated the carbon source precursor and found that the cost of Anthracite is low, and the preparation of Amorphous carbon anode material with Anthracite will help greatly reduce the battery cost. Through experiments, Anthracite based Sodium-ion battery anode material was finally developed. With Anthracite as precursor, it can reach 150-300Ah/yuan, which has significant cost performance advantages over other precursors.
The information provided in this article is for general guidance and information purposes only, and the content of this article should not be considered as investment, business, legal or tax advice under any circumstances.




 Phone:+86 13861313805
Phone:+86 13861313805
 E-mail:louis.han@e-tygroup.com
E-mail:louis.han@e-tygroup.com
 Add:Room 2809, Building Ⅱ, The Gate of the Orient, No. 199 Xinggang Street, Suzhou Industrial Park, Suzhou, China.
Add:Room 2809, Building Ⅱ, The Gate of the Orient, No. 199 Xinggang Street, Suzhou Industrial Park, Suzhou, China.
